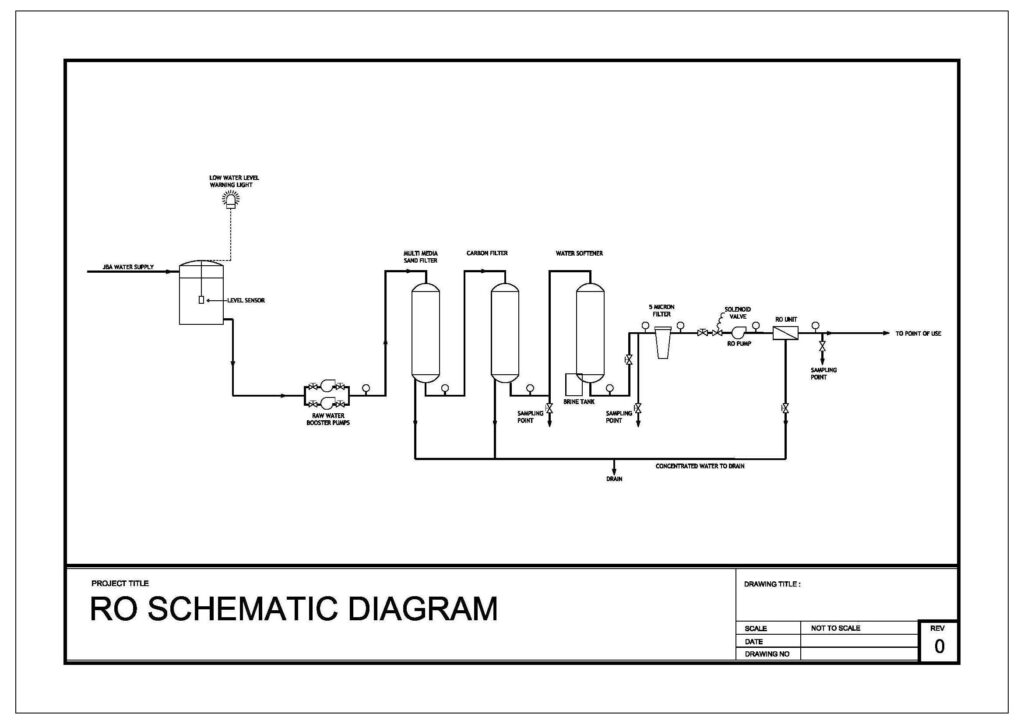
Reverse Osmosis System
Standard Features
-
TFC RO Membranes with 99% salt rejection
-
Reverse Osmosis System – Stainless Steel Frame
-
Automatic Feed water Shut Off Valve
-
Concentrate Pressure Gauge
-
Full Stainless Steel Raw Water Pump
-
Product Water Flow Meter
-
Concentrate Water Flow Meter
-
Pressure Regulating Valve
-
Stainless Steel RO Pump
-
Low Pressure Shut Off Switch
-
Reverse Osmosis System – Conductivity Monitor
-
Brackish Water Membrane
-
High-Flow Membrane
-
Alarm Event
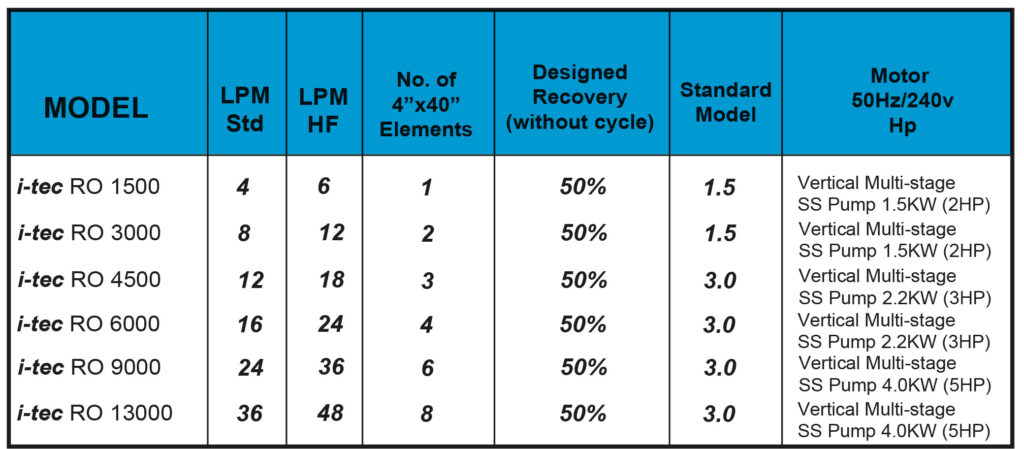
How does Reverse Osmosis work?
Reverse osmosis is a pressure driven process that forces water through a semi-permeable membrane that only passes the solvent (or water). The solute or larger molecules are concentrated and flushed out in the reject stream. RO removes approximately 99+% of dissolved salts in water. To understand the concept of reverse osmosis, recall the process of osmosis. Everything in nature wants to reach equilibrium. If there were two bodies of water separated by a semi-permeable membrane with different salt concentrations, they will reach equilibrium through osmosis; the solvent or water moves naturally through the membrane from the low salt concentration side to the high salt concentration side to balance (put in equilibrium) the concentration. Reverse Osmosis uses pressure to ‘reverse’ the natural flow of the permeate back to the reject stream.
TDS or ‘Total Dissolved Solids’ is a measure of the amount of salts or dissolved ions in water. It is the weight of remaining salts after the water has been filtered and the water evaporated, measured in mg/l which is equivalent to ppm or parts per million. The TDS or amount of salt in water varies from close to zero to seawater which is 36,000 ppm, depending on the region, higher TDS levels are seen in the Persian Gulf (up to 45,000 ppm). As a rule of thumb, 10 PSI per 1,000 ppm of TDS contributes 10 PSI of osmotic pressure. Therefore the higher the TDS, the higher pressure to required to overcome the osmotic pressure. Again Reverse Osmosis removes dissolved ions (salts), rejection is 99%+ depending on the membrane and specific ions. The WHO guideline or drinking water standard is 500 ppm.
Reverse Osmosis membranes are sheets rolled into a spiral. Cross flow technology is utilized to flush out the concentrated solutes. Water flows over the surface of the membrane, the solutes or salts are rejected while the solvent is forced through the membrane into the permeate spacer. The recovery is a function of the scaling potential of the feed water. RO systems do not run at 100% recovery as the solutes would precipitate out (form a solid), similar to the scale found on shower heads, kettles, etc. For example, if the feed is 1000 ppm TDS and the system runs at 75%, the reject would be approximately 4000 ppm and permeate stream 10 ppm.
In addition to anthropogenic sources of contaminated water, there are ‘naturally’ contaminated sources of water such as well water with high arsenic levels. While water is plentiful, over 97% is too salty for human consumption. Desalination utilizes the unlimited supply of seawater and through Reverse Osmosis, creating potable water by removing the salts. In addition to potable water, there are several other applications: food industry, car washing, boiler feed water, etc.
Reverse Osmosis systems consist of an intake supply of water, pre-treatment filters (screen and multimedia filters), Reverse Osmosis (RO) membranes and chemical dosing systems. Pre-treatment is required to remove any suspended solids before being pumped to the RO. The RO membranes remove dissolved solids only, splitting the feed water into permeate (potable water) and reject (concentrated salts). The process of Reverse Osmosis requires significant feed pressure depending on the TDS and temperature. The feed pressure ranges from 75-900 PSI. In order to describe Reverse Osmosis, it is first necessary to explain the phenomenon of osmosis. Osmosis may be described as the physical movement of a solvent through a semi-permeable membrane based on a difference in chemical potential between two solutions separated by that semi-permeable membrane.
The following example serves to demonstrate and clarify this point. A beaker of water as shown in figure 1 is divided through the center by a semi-permeable membrane. The black dotted line represents the semi-permeable membrane. We will define this semi-permeable membrane as lacking the capacity to diffuse anything other than the solvent, in this case water molecules.
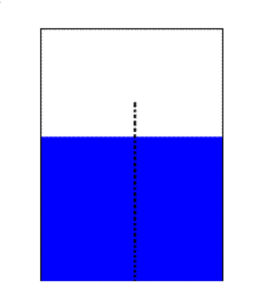
Figure 1. Reverse Osmosis Diagram
Now we will add a little common table salt (NaCl) to the solution on one side of the membrane (see figure 2). The salt water solution has a lower chemical potential than the water solution on the other side of the membrane. In an effort to equilibrate the difference in chemical potential, water begins to diffuse through the membrane from the water side to the salt water side. This movement is osmosis. The pressure exerted by this mass transfer is known as osmotic pressure.
The diffusion of water will continue until one of two constraints is met. One constraint would be that the solutions essentially equilibrate, at least to the extent that the remaining difference in chemical potential is offset by the resistance or pressure loss of diffusion through the membrane. The other constraint is that the rising column of salt water exerts sufficient hydrostatic pressure to limit further diffusion. By observation then, we can measure the osmotic pressure of a solution by noting the point at which the head pressure impedes further diffusion.
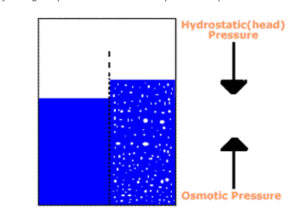
Figure 2. The osmosis process at work
By exerting a hydraulic pressure greater than the sum of the osmotic pressure difference and the pressure loss of diffusion through the membrane, we can cause water to diffuse in the opposite direction (Figure 3), into the less concentrated solution. This is reverse osmosis. The greater the pressure applied, the more rapid the diffusion. Using reverse osmosis we are able to concentrate various solutes, either dissolved or dispersed, in a solution.
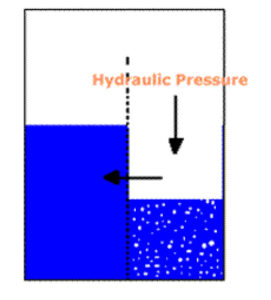
Figure 3. Hydraulic Pressure Causing Reverse Osmosis